eProtocol Automation
Fully Finished, Compliant Protocols in an Hour
AI-Powered. Collaborative. Compliant.
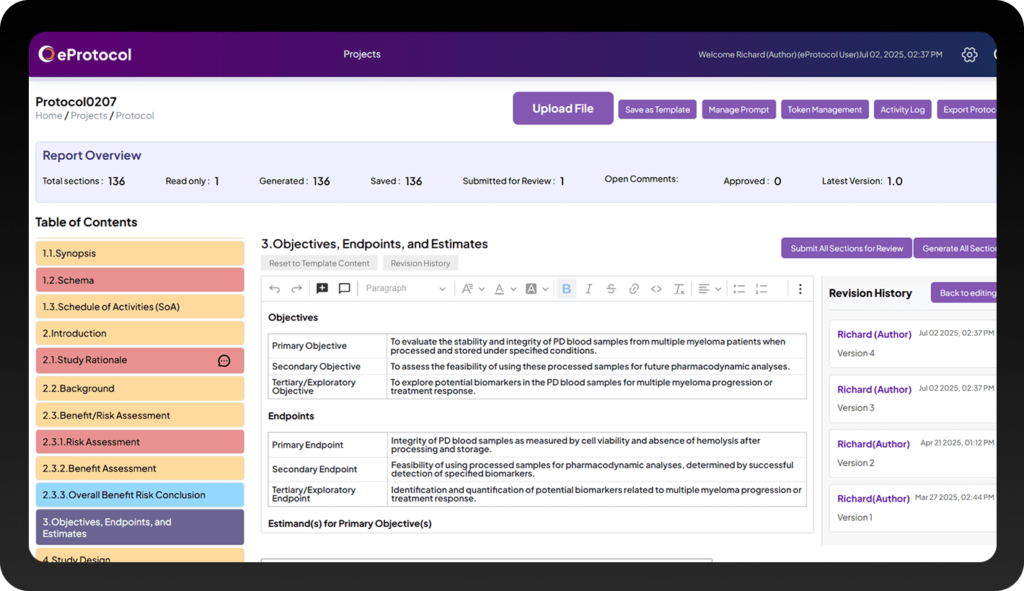
Results
Transform Clinical Trials Protocol Creation with Measurable Results
Clinion’s eProtocol Generation transforms how medical writing teams work, enabling them to produce a complete clinical trials protocol with greater speed and guaranteed compliance—all while reducing costs, simplifying collaboration, and maintaining perfect, audit-ready traceability.
Faster Protocol Development
Generate up to 90% of your fully-finished clinical protocol design in hours, reducing weeks of manual effort.
Regulatory Compliance
Ensure your clinical trials protocol is compliant with ICH M11, TransCelerate, and sponsor-specific templates from the start.
Cost and Time Savings
Save up to 60% on resources and timelines with automated AI generation.
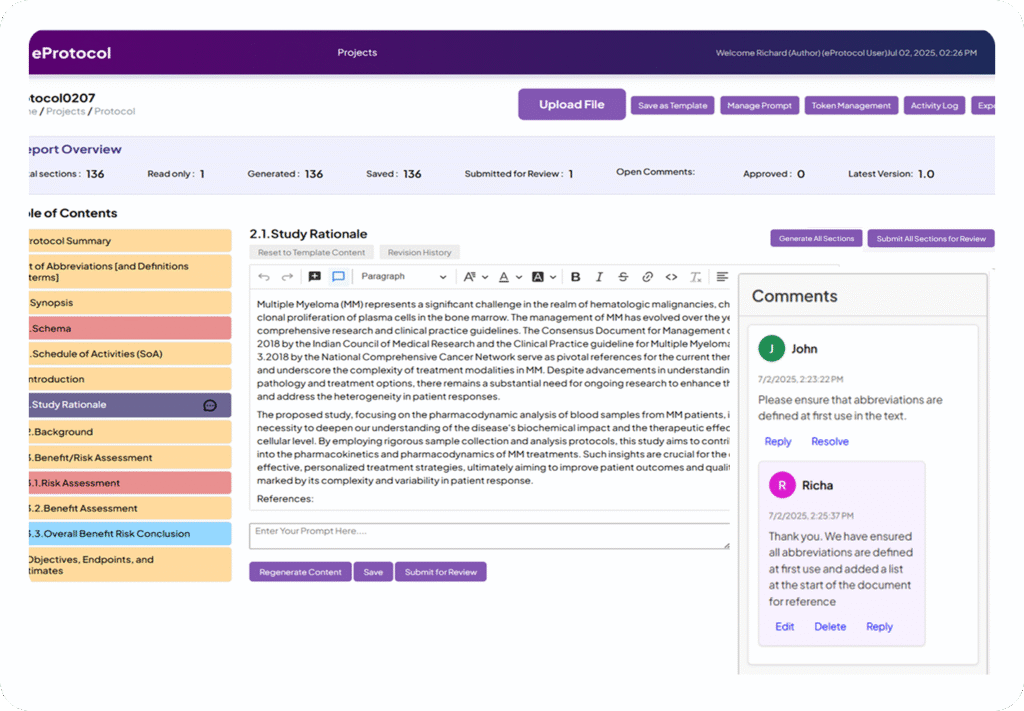
Streamline Your Clinical Trials Protocol Workflow in Four Steps
Upload Your Summary
Provide a brief protocol summary along with reference documents. AI analyzes the input, extracting key details.
Generate a Compliant Protocol
AI creates a structured clinical trials protocol draft aligned with TransCelerate and ICH guidelines, using sample client protocols, a trained knowledge base, and trusted third-party sources.
Collaborate in Real Time
Medical writers and reviewers edit and comment on the eProtocol draft with full version tracking.
Finalize and Export
Reviewers finalize the clinical trials protocol, ensuring completeness, then export it as a Word document.
Why Clinion?
Why Choose Clinion eProtocol for Your Clinical Trials?
Purpose-built for medical writing teams, Clinion eProtocol combines cutting-edge AI with regulatory compliance to transform your clinical protocol design and development process.
Pioneering AI Automation
Accelerate your trials with AI-driven protocol drafting and clinical trial protocol optimisation that’s faster than traditional methods.
Designed for Medical Writing Teams
Streamline end-to-end clinical trials protocol development with a specialized solution for efficient, compliant, error-free drafting.
Secured and Compliant by Design
Ensure secure, transparent, and compliant clinical trials protocol with ethical AI practices tailored for clinical trials.
Interactive Demo
Explore eProtocol
eProtocol Guided Walkthroughs
Take a guided tour through our end-to-end workflow from each key perspective. See how Admins effortlessly set up projects and manage users, while a Medical Writer uses AI to instantly generate a structured protocol draft. Then, step into the Reviewer’s role to add inline comments, track revisions, and approve content, creating a seamless collaborative cycle that produces a compliant, submission-ready protocol faster than ever.
FAQS
Frequently Asked Questions
Discover quick solutions to your Clinion eProtocol platform queries
Designing a clinical trial protocol starts with defining the study objective and choosing the right trial phase and population. Next, inclusion/exclusion criteria, treatment arms, randomization, and endpoints are set. The protocol must detail visit schedules, safety monitoring, and data collection plans. It’s finalized after input from medical, regulatory, and biostatistics teams to ensure compliance and feasibility.
A clinical trial protocol is a regulatory document that outlines how the trial will be conducted, including objectives, methodology, and safety procedures. A study plan, often used internally, may include operational or logistical details not required for regulatory submission. While both guide trial execution, the protocol is legally binding and subject to regulatory review.
Clinion eProtocol uses AI to generate ICH M11 and TransCelerate-compliant drafts in minutes. It automates up to 90% of the writing process - drafting, review, collaboration, and finalization, on a single platform. This reduces costs by up to 60%, improves consistency, and ensures regulatory compliance.
Yes, the workflow is designed to keep medical writers in control. After the AI generates the initial draft, writers can review, refine, and adjust the content as needed. This ensures the final document reflects both the study’s intent and the team’s preferences, while maintaining speed and structural integrity.
Clinion’s AI is trained on a diverse set of real-world clinical trial protocols from multiple therapeutic areas, geographies, and trial phases. It uses a curated, regulated knowledge base to ensure balanced, evidence-based outputs. The system is designed to follow industry standards, not personal or commercial bias, making it a reliable, objective support tool for protocol drafting.
Still have questions?
Explore how Clinion AI can accelerate your trial – reach out to our team.
Responsible AI
Clinion follows Responsible AI principles, ensuring its AI tools are built for safety and reliability, and remains committed to Data Privacy and Security at every step.
- Accountability
- Transparency
- Privacy & Security
- Reliability & Safety
- Fairness
Unlock the Future of Clinical Trials with Clinion.
Cut your trial costs by 35% and accelerate your time-to-market by 30%
Compliance
Fully Compliant with Global Standards


