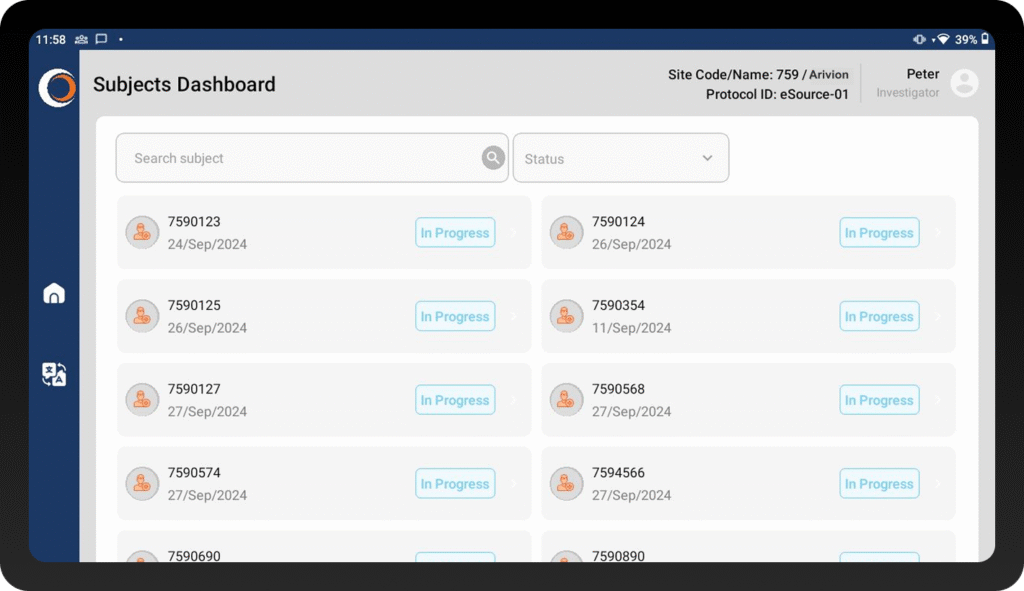
Why Clinion?
Proven Impact from First Data Point to Database Lock
Bottlenecks in clinical trials often start at the source – the way data is captured and entered. Manual data entry processes slow progress and introduce unnecessary risks, including transcription errors, missing information, and compliance challenges. Clinion’s eSource software removes these barriers by enabling site users to enter source data directly into a secure mobile app, capturing it at the point of care. This ensures that the data is not raw or scattered but transformed into a single, reliable source of truth – where every datapoint is accurate, complete, and ready for immediate use.
Faster Study conduct
Electronic direct-data-capture (DDC) removes the need for duplicate entry and lengthy data cleaning cycles. This means high-quality datasets are ready from the first patient visit, accelerating database readiness and enabling earlier trial milestones.
Reduced Monitoring Costs
With real-time quality metrics and automated data validation, CRAs can prioritize high-risk datapoints instead of performing exhaustive site-wide reviews. This targeted approach cuts down on unnecessary site visits and reduces overall monitoring spend.
Full Regulatory Confidence
Every datapoint is electronically traceable with timestamps, audit trails, and version control. Our eSource system fully meets FDA 21 CFR Part 11, GDPR, and ICH-GCP requirements, ensuring your trial data is always inspection-ready for global regulatory bodies.
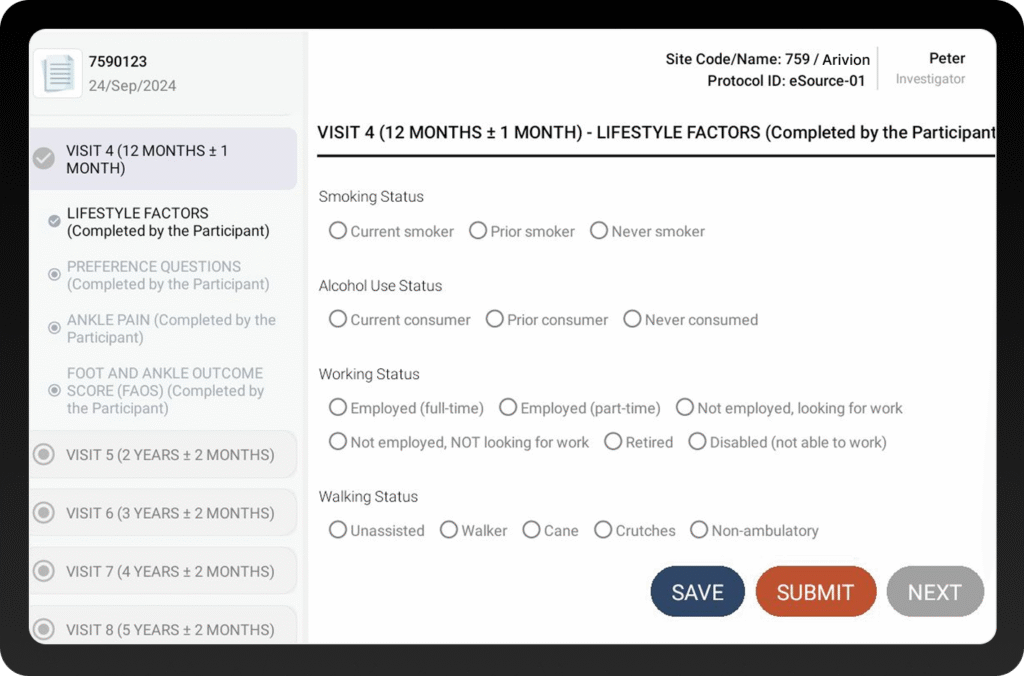
From Source Document to Decision-Ready Data
Instant Data Entry
Site teams capture data directly into the system, eliminating delays and transcription errors.
Seamless EDC Integrationg
eSource forms sync directly to the EDC in real time, eliminating the need for mapping and ensuring consistent data flow.
Regulatory-Ready & Fully Auditable
Every entry is time-stamped, securely logged, and fully traceable for compliance.
Remote Monitoring & Oversight
Sponsors and CRAs access data from anywhere, reducing site visits and accelerating decision-making.
Features
Advanced Capabilities That Power Better Outcomes
Direct Data Capture (DDC)
Site personnel enter trial data directly into eSource-enabled devices at the point of care, ensuring that every entry reflects the exact context in which it was collected.
Easy-to-Use Interface
Simplifies data entry and navigation with a clean, intuitive interface. Its user-friendly design helps reduce errors and speeds up the process, making it easy for anyone to use without extensive training.
Configurable User Permissions
Allows precise control over who can access and edit data within the eSource platform, enhancing security and ensuring compliance with regulatory requirements.
Interactive Demo
Explore eSource

eSource Guided Walkthroughs
Take an interactive walkthrough of the Clinion eSource workflow from key user perspectives. The process begins when site staff enter patient data directly into the Clinion eSource platform during the visit, capturing information instantly. Next, see how the system uses intelligent parsing and automated validation to ensure data accuracy, while CRAs review and verify the information in real time. Finally, watch how verified data flows seamlessly into Clinion EDC, creating a centralized, compliant, and up-to-date source of truth accessible to all stakeholders.
FAQS
Frequently Asked Questions
Discover quick solutions to your Clinion eSource platform queries
eSource is a broader approach to collecting source data electronically, while eCOA and ePRO are specific types of eSource. ePRO captures direct patient input (e.g., symptoms or quality of life), and eCOA includes clinician, observer, and performance assessments. All are forms of eSource but focus on different types of data input during trials.
Clinion eSource integrates seamlessly with Clinion EDC and can connect with other clinical systems. It allows real-time data to flow directly from the source into centralized platforms, reducing delays and ensuring a single source of truth. This interoperability improves trial coordination and minimizes manual data transfers across systems.
Yes, Clinion eSource is fully compliant with FDA 21 CFR Part 11, GDPR, and other global regulations. It features secure user access, automated audit trails, version control, and time-stamped entries, ensuring transparency, traceability, and data integrity throughout the trial. This helps sponsors and sites stay inspection-ready at all times.
Yes, PIs can securely access and review trial data from anywhere. Clinion eSource provides remote oversight tools that help PIs approve or monitor forms without being on-site. This improves responsiveness, reduces delays, and supports better compliance across busy multi-site trials.
Yes, Clinion eSource supports offline data entry. Site teams or patients can input data without an internet connection, and it will automatically sync with Clinion EDC once connectivity is restored. This ensures uninterrupted data collection in remote locations or during connectivity outages.
While EMRs (Electronic Medical Records) are designed for routine patient care, eSource systems are built specifically for capturing clinical trial data in compliance with GCP and regulatory standards. eSource structures and formats data for trial-specific protocols, while EMRs focus on general healthcare documentation and aren’t optimized for research workflows.
Still have questions?
Explore how Clinion AI can accelerate your trial – reach out to our team.
Featured Resources
Dive deeper into Clinical Trial Innovation
Sarah is responsible for overseeing a complex clinical trial with multiple sites around the world.
Learn how Clinion’s AI-powered EDC boosts trial efficiency through automation from study setup to final reports.
Clinion’s AI-powered Clinical Data Review is recognized as a breakthrough innovation in clinical trial technology.
Clinion enabled India’s first COVID-19 vaccine trial launch in record time, achieving FPI in just 3 weeks during the pandemic.
Responsible AI
Clinion follows Responsible AI principles, ensuring its AI tools are built for safety and reliability, and remains committed to Data Privacy and Security at every step.
- Accountability
- Transparency
- Privacy & Security
- Reliability & Safety
- Fairness
Unlock the Future of Clinical Trials with Clinion.
Cut your trial costs by 35% and accelerate your time-to-market by 30%
Compliance
Fully Compliant with Global Standards





