Why Clinion
Optimize Management, Ensure Full Compliance
Our electronic Trial Master File (eTMF) is a comprehensive, secure, and scalable platform designed to optimize your document management. It ensures full regulatory compliance and facilitates seamless collaboration, empowering your team with efficient oversight across the entire trial lifecycle.
Reduction in Study Reconciliation Time
Clinion eTMF’s real-time tracking and automated document oversight drastically cut reconciliation efforts, speeding up your study closeout.
Savings in Admin Costs
Our intuitive eTMF software reduces the burden on administrative teams, lowering operational costs and enhancing trial efficiency.
Inspection-Ready
Clinion eTMF guarantees you are always audit-ready, with automated alerts, real-time document tracking, and clear, traceable records of TMF activity.
From Documents to Total Readiness, Simplified.
Setup & Plan
Establish the framework for your eTMF clinical trials by defining user roles, selecting DIA or sponsor-specific standards, and creating a detailed TMF file plan with critical milestones.
Conduct & Collaborate
Upload, index, and manage all trial documents. A robust workflow guides documents through review and QC, with compliant e-signatures for final approval.l.
Monitor & Oversee
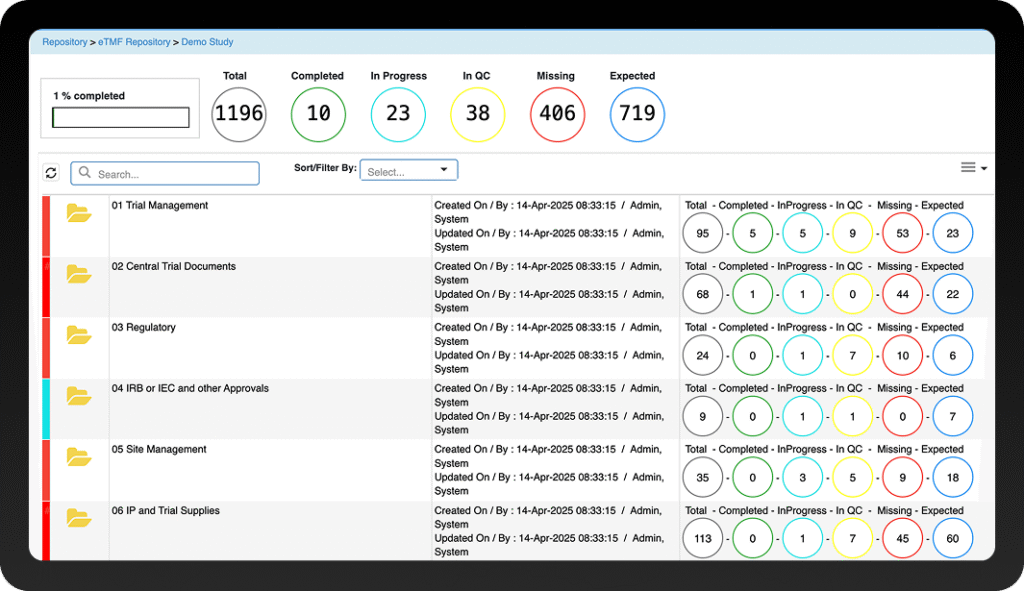
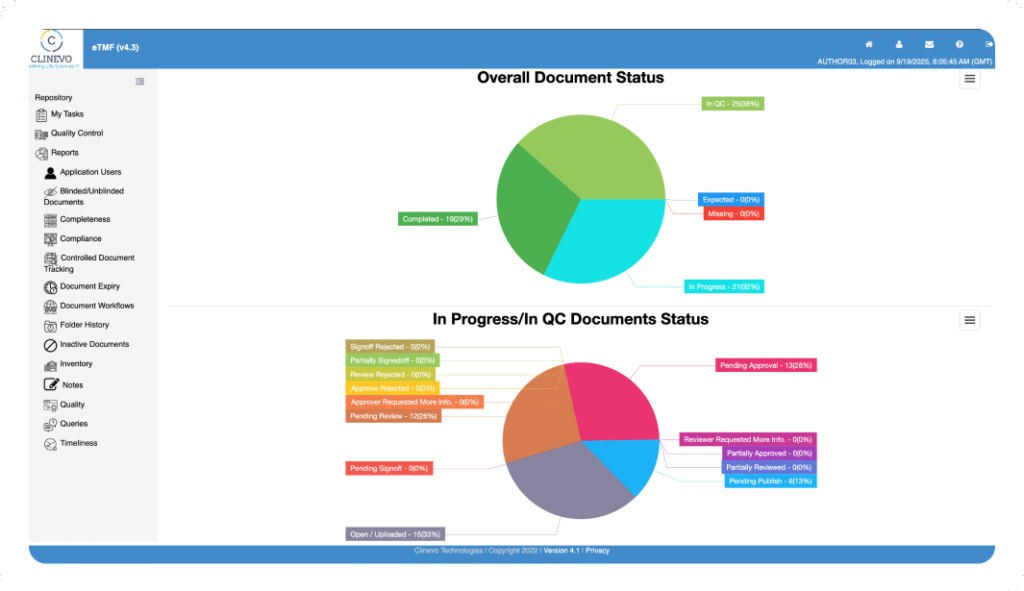
Maintain complete oversight with real-time dashboards. Instantly track key metrics like eTMF completeness, timeliness, quality, and overall study compliance through detailed reports.
Lock & Archive
Once the trial is complete, securely lock the eTMF system to prevent further changes. The eTMF system ensures your final documentation is prepared for compliant, long-term archival.
Features
Your Toolkit for Efficient Electronic Trial Master File Management
File Planning
Establish a clear roadmap for your trial from day one. Our platform lets you create detailed file plans and set critical milestones at the study, country, and site levels, ensuring everyone is aligned on eTMF expectations.
Live Tracking
Never fall behind on compliance. Our eTMF system automatically cross-references your file plan to provide live tracking of all expected documents, instantly flagging any that are missing or delayed so you can take immediate action.
Insightful TMF Analytics
Move from simply collecting data to making informed decisions. Our advanced analytics provide deep insights into eTMF health, with dashboards to monitor the completeness, quality, and timeliness of your Electronic Trial Master File, giving you a complete view of study compliance.
Flexible TMF Structures
Our Electronic trial master file software adapts to your trial, not the other way around. Start with the prebuilt DIA reference model for industry-standard compliance, or easily configure a custom, sponsor-specific structure to meet your unique study requirements with our eTMF.
FAQS
Frequently Asked Questions
Discover quick solutions to your Clinion eTMF platform queries
An eTMF stores all essential clinical trial documents, including protocols, investigator brochures, informed consent forms, IRB/IEC approvals, safety reports, monitoring visit logs, regulatory submissions, contracts, and correspondence. It centralizes documentation for easy access, audit readiness, and alignment with the TMF Reference Model.
During inspections, auditors review document completeness, metadata accuracy, version control, and user access logs. A compliant eTMF supports this with real-time dashboards, audit trails, and a standardized structure that meets ICH GCP and 21 CFR Part 11 requirements.
Clinion eTMF can be launched in just a few days using its pre-built, DIA-compliant structure. It includes smart defaults, customizable templates, and an intuitive interface that eliminates setup delays and enables faster trial startup.
Yes, Clinion eTMF is built on the DIA TMF Reference Model and is fully configurable. Sponsors and CROs can customize folder structures, metadata fields, naming conventions, and workflows while maintaining compliance and audit readiness.
Clinion eTMF automates document tracking, sends smart reminders, and flags missing files through real-time dashboards. These features significantly reduce manual reconciliation efforts and can cut study closeout timelines by up to 40%.
Still have questions?
Explore how Clinion AI can accelerate your trial – reach out to our team.
Responsible AI
Clinion follows Responsible AI principles, ensuring its AI tools are built for safety and reliability, and remains committed to Data Privacy and Security at every step.
- Accountability
- Transparency
- Privacy & Security
- Reliability & Safety
- Fairness
Unlock the Future of Clinical Trials with Clinion.
Cut your trial costs by 35% and accelerate your time-to-market by 30%
Compliance
Fully Compliant with Global Standards


