Why Clinion?
Making Patient Data Collection Simple and Effortless
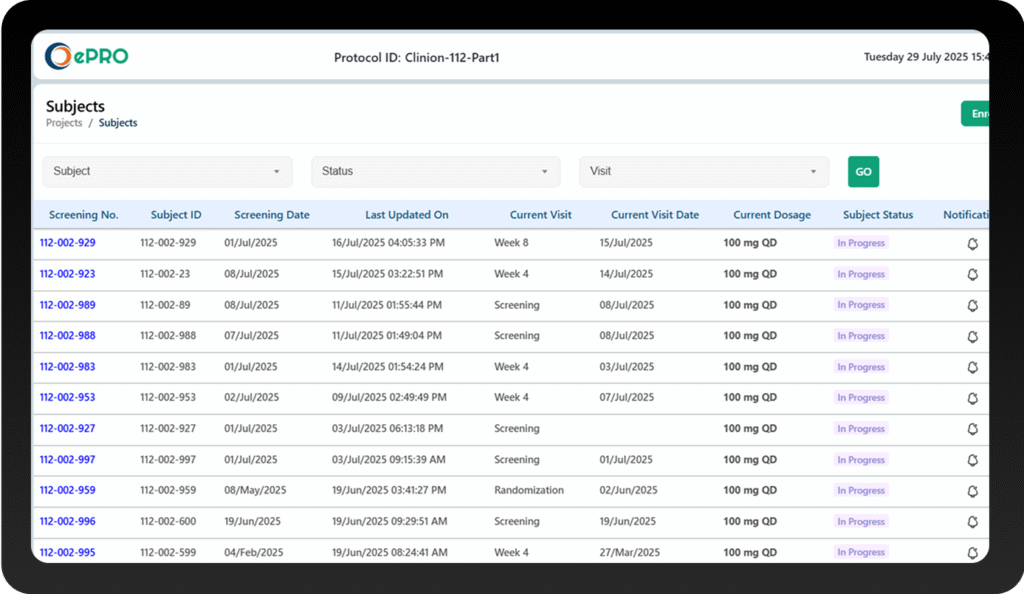
As regulatory focus on real-world data (RWD) and real-world evidence (RWE) grows, the demand for a flexible and robust ePRO software is more critical than ever. Clinion’s Electronic Patient Reported Outcome (ePRO) rises to this challenge with a secure, compliant platform that captures patient-reported outcomes in real time through an easy-to-use mobile app on their own devices, capturing responses directly from patients without intermediaries. This direct data flow delivers valuable real-world evidence for deeper insights in your ePRO clinical trials.
Fully Compliant
Clinion’s ePRO system meets key regulatory standards, including FDA 21 CFR Part 11, GDPR, and ICH-GCP, featuring role-based access and tamper-proof audit trails for secure and compliant data capture.
Scalable for Global Trials
Clinion ePRO supports your study setup in two ways: as a Standalone platform or when Integrated with Clinion EDC. Standalone securely captures patient-reported data independently, while Integration ensures seamless data flow for streamlined trials.
Designed for Flexibility
Proven across studies involving over 14,000 patients, Clinion’s ePRO software scales effortlessly from single-site to multi-regional trials. Accessible via iOS, Android, and web platforms, it ensures reliable performance and efficient data management anytime, anywhere.
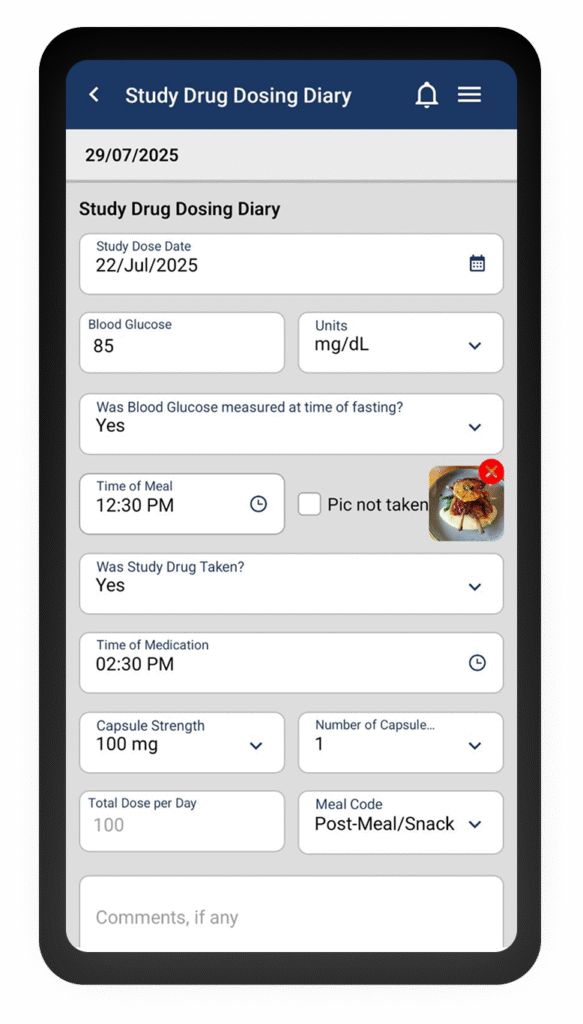
Capture Patient Data Seamlessly
Study Setup
We configure and customize your ePRO forms quickly with multilingual support, making them ready for patient use.
Patient Data Collection
Patients submit responses through the Clinion ePRO app on their mobile devices, with automated reminders to ensure timely completion.
Real-Time Validation & Syncing
Data entries are validated instantly for accuracy and synced securely with Clinion EDC or stored safely in standalone mode.
Ongoing Monitoring
Clinical teams access real-time patient data through our ePRO system dashboards for immediate review and decision-making.
Complete Audit Trail
All data changes are logged automatically to maintain regulatory compliance and data integrity.
Features
Features That Empower Patients and Enhance Data Quality
Effortless Patient Experience
The Clinion ePRO system features a very easy-to-use, multilingual interface with no learning curve. Automated reminders and push notifications keep patients engaged and on track across all devices.
Freedom to Use Any Device
With a BYOD model, patients report Electronic Patient Reported Outcomes using their own smartphones, tablets, or computers (iOS, Android, or web browsers), removing the need for extra devices and hence increasing convenience.
Flexible Forms with Trusted Questionnaires
Our team quickly sets up and customizes patient surveys in multiple languages. This ePRO solution includes trusted, licensed health questionnaires like EQ-5D-5L and PROMIS, designed to fit your study’s specific need
Smart AI-Powered Validation
Real-time field validations combined with AI-driven comparison of ePRO and EDC data detect discrepancies early, reducing manual queries and improving overall data quality.
Interactive Demo
Explore ePRO

ePRO Guided Walkthroughs
Take an interactive walkthrough of the Clinion ePRO workflow from key user perspectives. The process begins when a site-level user enrolls a patient in Clinion EDC, triggering an automatic email and mobile notification to the patient. From there, step into the patient’s experience as they install the application on their smartphone or tablet and begin reporting health outcomes in real time. Finally, see how clinicians gain a comprehensive view of patient progress, with all reported data automatically synced to the EDC for immediate access and review.
FAQS
Frequently Asked Questions
Discover quick solutions to your Clinion ePRO platform queries
eCOA (electronic Clinical Outcome Assessment) includes data reported by patients (ePRO), clinicians (ClinRO), caregivers (ObsRO), and performance-based outcomes (PerfO). ePRO is a subset of eCOA, focused solely on patient-reported outcomes. eCOA offers a broader view by capturing inputs from multiple sources.
ePRO software enables patients to report health outcomes via mobile apps or web platforms. It guides them through validated assessments and securely transmits data to the trial database in real time. This improves data quality, reduces site workload, and enables the collection of reliable real-world data (RWD) for regulatory-ready submissions.
Yes, Clinion ePRO is built to scale. It has supported global studies with over 20,000 patients and offers a robust infrastructure for seamless data collection across both small and multi-site, large-scale trials.
Yes, Clinion ePRO can integrate with other EDCs, provided the other system offers accessible APIs. The integration is secure, compliant with global standards like HIPAA and GDPR, and ensures encrypted, audit-ready data transfer.
No, ePRO only captures outcomes reported directly by patients. However, broader eCOA platforms include caregiver-reported (ObsRO) and clinician-reported (ClinRO) assessments, allowing for a more integrated and complete view of patient outcomes in clinical research.
Still have questions?
Explore how Clinion AI can accelerate your trial – reach out to our team.
Responsible AI
Clinion follows Responsible AI principles, ensuring its AI tools are built for safety and reliability, and remains committed to Data Privacy and Security at every step.
- Accountability
- Transparency
- Privacy & Security
- Reliability & Safety
- Fairness
Unlock the Future of Clinical Trials with Clinion.
Cut your trial costs by 35% and accelerate your time-to-market by 30%
Compliance
Fully Compliant with Global Standards


