EDC
The Only True
AI-Native EDC Platform
Drive down clinical trial costs while ensuring superior data quality, faster study builds, and optimal efficiency with electronic data capture software
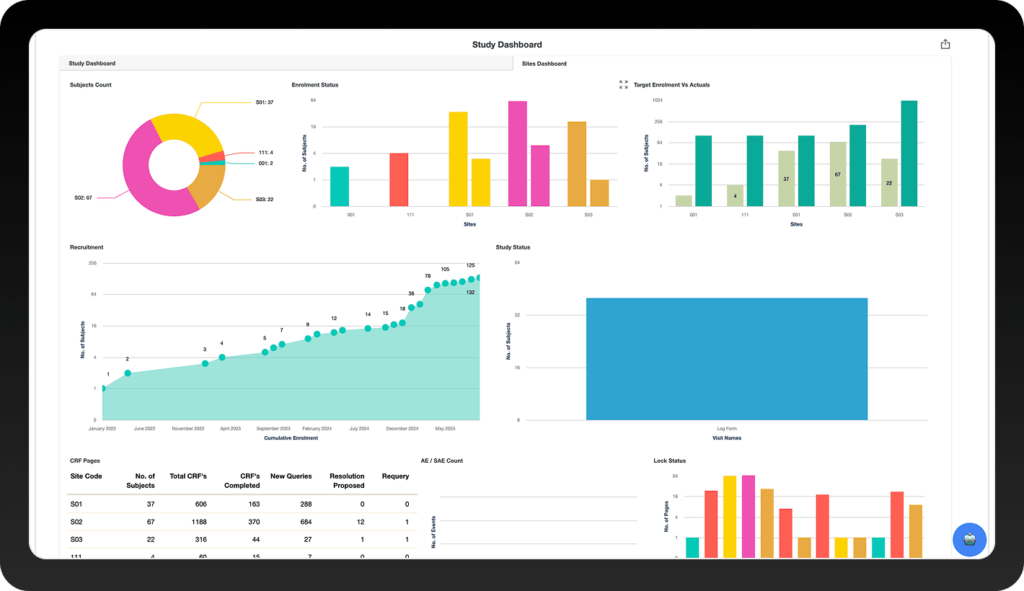
Why Clinion EDC?
Join 100+ Customers Accelerating Trials Across Phase I to IV
Countries
Subjects
Customers
Clinical Trials
Biotech Studies
FDA Studies
Industry-Leading AI Capabilities
Clinion offers the most extensive AI suite in clinical trials, enabling accelerated study builds, improved data management and review, and a smoother study close-out, resulting in enhanced data quality & efficiency, and reduction in overall trial costs.
Unified & Seamless Integration
Unified Data Ecosystem ensures seamless integration with key modules: RTSM, ePRO, CTMS, eConsent, and eSource, enabling a streamlined user experience and consolidated data visibility.
Fastest Study Setup in the Industry
Clinion provides the industry’s fastest study build through codeless standardized global libraries, enabling configuration in less than two weeks.
Features
Key Features of our Electronic Data Capture Software
ACCELERATE STUDY SETUP
Deploy Studies in Weeks, No Programming Required
Clinion’s Standardized Global Library offers prebuilt, CDASH-compliant, therapeutic area-specific templates that enable rapid study configuration. Users can instantly duplicate forms and selectively customize edit checks, skip logics, or bulk edit configurations as needed. This approach eliminates manual programming needs while providing greater flexibility.
2-4 Weeks
Average Go-Live Timeline
50%
Reduction in Operational Build Costs
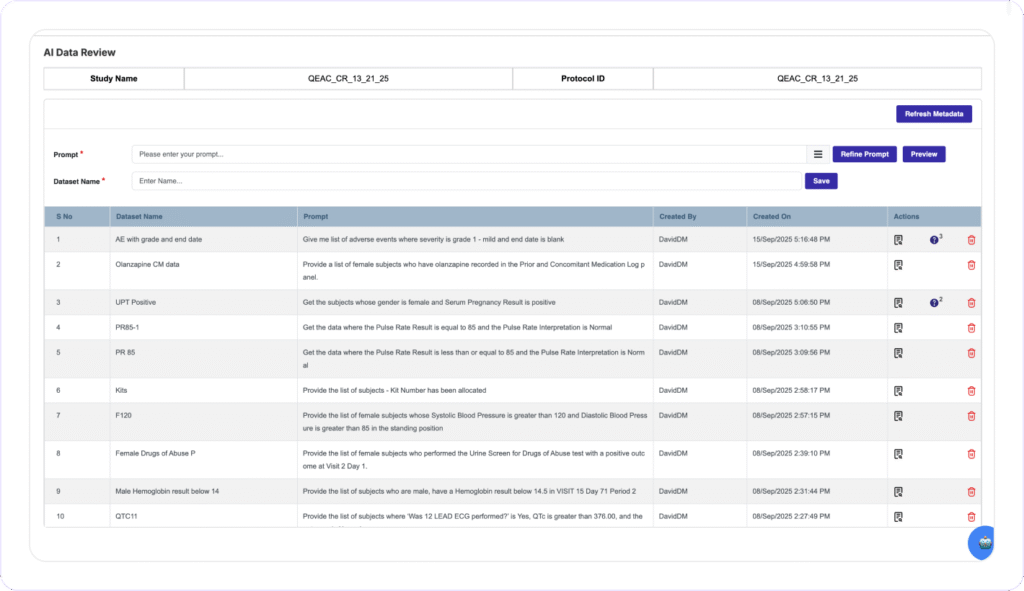
NO MORE DATA DISCREPANCY
Instant Discrepancy Detection and Resolution
Clinion’s Award-winning AI Data Review allows Data Managers to create custom datasets on the fly using Natural Language prompts and identify and flag data discrepancies directly on the EDC system. Data Managers can issue bulk queries or corrections across all affected subjects with one action, eliminating Biostats dependencies and long review cycle times.
40-50%
Faster Time to Database Lock
40-50%
Savings in Total Data Management Costs
POST-PRODUCTION CHANGES IN REAL-TIME
Manage Protocol Amendments without any Downtime
Mid-Study Changes can be applied to live studies without any downtime. You can add, edit, or delete visits, pages, or fields after launch without vendor assistance. Existing data stays safe through automatic version control. When you update forms, locks release instantly. Your reports always pull from the latest CRF version, eliminating manual reconciliation work.
0%
Study Downtime During Protocol Updates
40%
Savings in time and costs
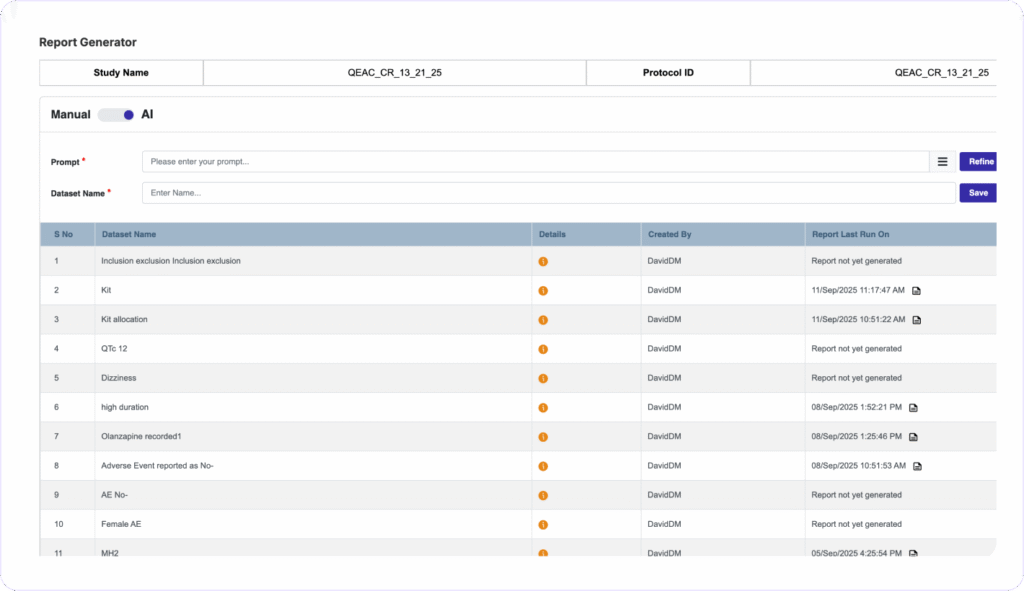
AUDIT-READY REPORTS IN SECONDS
Generate Custom Reports Instantly, Zero Coding Needed
Clinion’s AI Report Generator turns natural language prompts into tailored reports and does not require any programming skills. You can schedule automated notifications to stakeholders, maintain full audit trails with version history, and push data to third-party tools via secure APIs.
80-90%
Faster Report Generation vs Manual
75-85%
Reduction in Reporting Costs
AUTOMATED CDISC COMPLIANCE
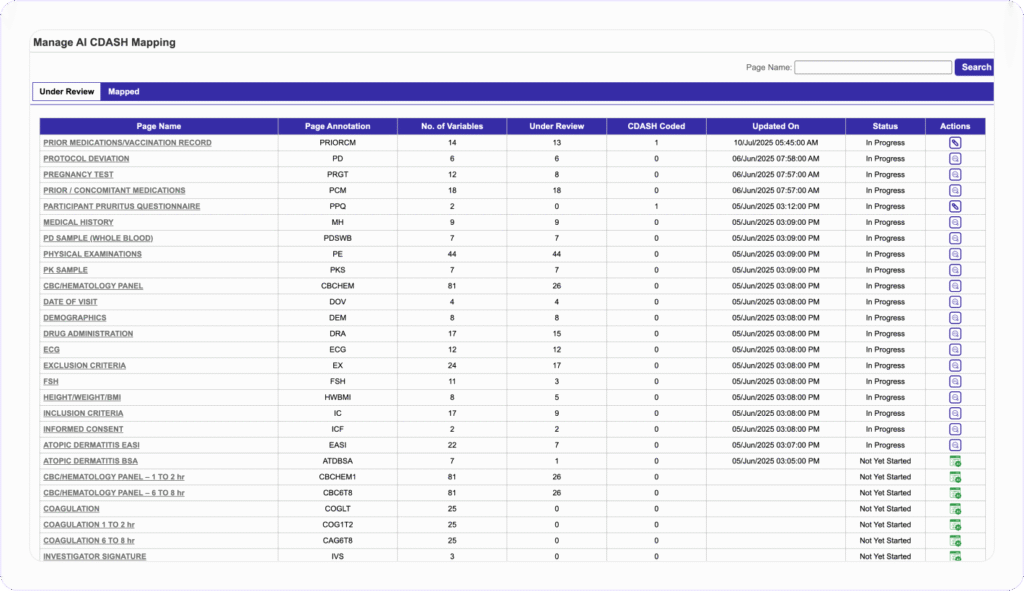
Map Variables to CDASH
Standards in Minutes, Not Weeks
Clinion’s AI CDASH Mapping converts custom annotations to CDASH standards with one click. Users select a study page and send variables to the AI engine to instantly receive annotated mapping suggestions. They can then review, accept, customize, or check against CDASH libraries or apply their own annotations, all in a single workflow.
40%
Coding Effort Savings
20%
Study Setup Time as per Standards
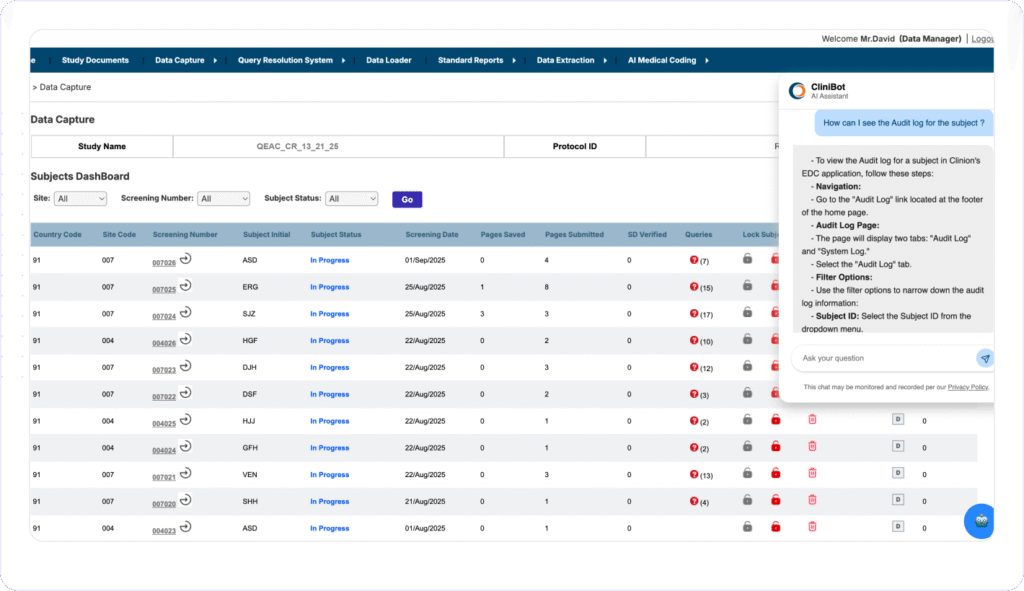
INSTANT STUDY SUPPORT with AI
Resolve issues Fast,
No External Help
Clinion’s CliniBot is an AI-Conversational Bot that interacts via chat, responding to user queries in natural language. Trained on Clinion EDC protocol and user manuals, it can guide users through tasks, clarify CRF fields, explain product features, and provides contextual help across modules, reducing errors and improving study quality.
- Provides immediate, in-platform assistance to users without needing external support.
- Reduces support ticket volume and eases the burden on training and help desk teams.
- Increases user satisfaction and accelerates platform adoption across teams and studies.
Interactive Demo
Explore EDC Software
EDC Guided Walkthroughs
Clinion EDC system is built with AI at its core, not as an add-on. Automate key steps of your study, including Study Setup, CDASH mapping, Remote Source Data Verification, AI Medical Coding, Data Review, and Custom Reporting, with intelligent modules that work seamlessly together. Clinion EDC is built to accelerate trials, with greater efficiency and quality at reduced costs.

Testimonials
What Clinion Customers Are Saying
Find out why Clinion EDC has a 4.6 rating in G2
The user friendly EDC
Clinion EDC is easily accessible. It has a good adaptability nature for panels, which makes it trouble-free for all users. Redirecting to different pages effortlessly makes this EDC a big choice for everyone.
Dr. Sahaja K.
Clinical Research Associate
Most easy to use and faster to setup
For the past 2 years , I have used Clinion EDC and I find myself very satisfied with how user friendly it is, even for individual lacking software technical knowledge.
Naga T
Executive – Clinical Data Analyst
Easy to set up screen and rule
I have been using Clinion EDC for the past 8 years, and its easy to use interface, even for users with limited technical skills. Easy to setup screen in one day.
Ravi Shanker K
Deputy General Manager, Pharmaceuticals
Good experience with Clinion EDC
The team was very helpful and approachable with all the changes that were required. They took us through all the tabs very patiently and explained all sections.
Shruti K
Mid-Market
Good user interface and easy to learn
It’s easy comparative to other tools for data capture and first time users can also understand easily while using this EDC tool. EDC integrated with IWRS is very helpful for randomization.
Gajapathiraju p.
Clinical Data Manager
FAQS
Frequently Asked Questions
Discover quick solutions to your Clinion Electronic Data Capture (EDC) system queries
Clinion EDC enhances accuracy and efficiency through AI-powered edit checks that identify errors in real time and intelligent workflows that automate repetitive tasks. This reduces manual errors by up to 30% and enables faster database lock. Built-in reporting provides deeper insights for CROs and sponsors.
Clinion EDC is built with AI at its core. It automates Study Setup, CDASH mapping, Remote SDV, Medical Coding, Data Review, and Reporting through integrated modules. This speeds up execution, improves data quality, and reduces costs across the study lifecycle.
Clinion’s AI-powered Data Review tool allows users to generate custom datasets simply by describing what they need in plain English. This eliminates reliance on technical teams and speeds up data access. Users can raise queries directly within the dataset, enabling faster reviews, quicker decisions, and higher data quality throughout the clinical trial process.
Clinion EDC has an AI-powered Data Reporter that helps you create custom datasets across sites, subjects, visits, and fields with just a few clicks. You can save, schedule, or email reports, and even connect them to third-party tools using APIs. With the built-in GenAI chart generator, you can create bar, pie, scatter, and other charts instantly and share them for faster, data-driven decisions.
Yes, Clinion EDC handles complex validations, protocol amendments, and bulk edits with ease. It supports mid-study updates without disrupting workflows, maintaining compliance and data integrity throughout the process.
Clinion EDC integrates seamlessly with CTMS, RTSM, and ePRO. This enables real-time data flow, eliminates silos, and simplifies end-to-end trial execution, improving operational efficiency and oversight.
Still have questions?
Explore how Clinion AI can accelerate your trial – reach out to our team.
Responsible AI
Clinion follows Responsible AI principles, ensuring its AI tools are built for safety and reliability, and remains committed to Data Privacy and Security at every step.
- Accountability
- Transparency
- Privacy & Security
- Reliability & Safety
- Fairness
Unlock the Future of Clinical Trials with Clinion.
Cut your trial costs by 35% and accelerate your time-to-market by 30%
Compliance
Fully Compliant with Global Standards


