Why Clinion ?
Building Patient Trust and Compliance
Traditional consent is slow, error-prone, and burdensome, impacting patient enrollment. Clinion electronic consent enhances engagement and compliance with digital access, real-time tracking, and seamless EDC integration for a patient-centric, audit-ready process. Our electronic informed consent software is offered in three models: Site eConsent, Remote eConsent (also known as virtual consent), and eConsent bundled with ePRO. It gives sponsors flexibility, sites control, and patients a smooth experience for faster and more compliant trials.
Faster Study Enrolment
Accelerate patient onboarding from months to weeks, and automate re-consenting for protocol amendments, avoiding delays or regulatory audit findings.
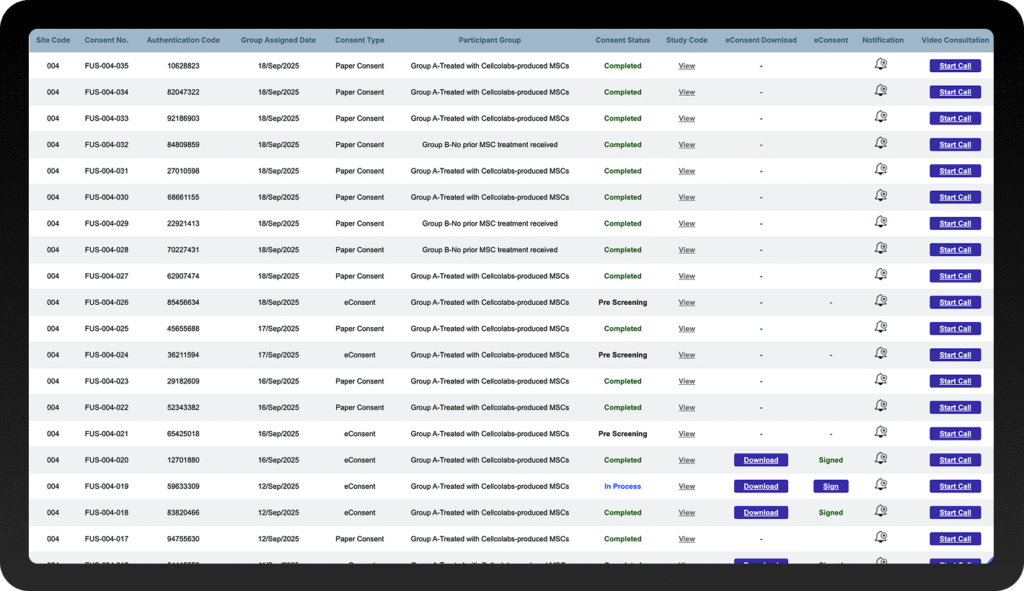
Complete Oversight with ICF Version Control
Auto-track consent versions, monitor patient onboarding in real-time, and maintain audit-ready logs with time-stamped signatures and compliance-proof documentation.
Increase Patient Comprehension & Retention
Enhance the patient onboarding experience with multimedia-driven, easy-to-understand consent workflows. Clear, accessible, and transparent information helps patients make informed decisions, builds trust, and keeps them engaged throughout the trial.

How It Works
From Consent to Compliance in Minutes
Patient Reviews the eConsent Form
Participants can access the digital eConsent form on their own devices for remote consent, or at the site on site-provided devices such as tablets and iPads.
Interactive & Informed Decision-Making
Patients read, ask questions, and digitally sign to ensure full understanding. For remote consent or when the eConsent software is bundled with ePRO, the principal investigator (PI) can engage with the patient through teleconsultation.
Seamless Site Oversight & Approval
Investigators review and approve consents instantly, eliminating paperwork delays.
Automatic EDC Enrollment
Subjects are automatically enrolled into the EDC system, with real-time visibility on the dashboard.
Automated Compliance Tracking
Time-stamped signatures, ICF version control, and regulatory compliance are effortlessly maintained throughout the process.
eConsent Benefits
Built for Every Stakeholder - One eConsent Software, Multiple Benefits
Intuitive & Engaging Patient Experience
A user-friendly UX with embedded questions and live support ensures clear understanding & compliance. The system also automates the delivery of digitally signed eConsent forms for secure record-keeping.
Full Flexibility &
Control
Ensure compliance and adaptability with remote editing and transparent version control, all backed by audit-ready documentation and no delays.
Integrated, Automated Enrollment
Automate subject enrollment and real-time data sync with built-in integration to Clinion’s EDC. Ensure all records are accurate, consistent, and reliable from the start.
Multilingual & Multi-Platform Accessibility
With multilingual support and seamless compatibility across iOS, Android, and tablet platforms, Clinion’s eConsent software ensures a smooth and consistent user experience worldwide.
FAQS
Frequently Asked Questions
Discover quick solutions to your Clinion eConsent platform queries
Clinion eConsent improves participant understanding through interactive features like videos, visual aids, and embedded FAQs. These tools support individualized learning and improve comprehension, helping reduce consent-related errors and ensuring true informed consent.
eConsent works seamlessly across interventional, observational, decentralized, and hybrid trials. It's built to support diverse study designs and populations, offering flexibility for both global and localized implementations, all while maintaining regulatory compliance.
Clinion eConsent integrates directly with Clinion EDC and can connect with other clinical systems. This enables real-time consent status updates, centralized data access, and more streamlined workflows, reducing manual effort and ensuring accuracy.
With Clinion eConsent, protocol amendments automatically trigger re-consent workflows. Participants receive notifications, and investigators can monitor who has re-consented in real time. This ensures both compliance and study continuity.
Clinion’s eConsent platform stores audit trails, manages version histories, and logs every action with time stamps. It is designed to meet FDA and GCP guidelines, ensuring full compliance with regulatory and sponsor requirements.
Participant data is protected through end-to-end encryption, role-based access controls, and compliance with HIPAA, GDPR, and 21 CFR Part 11. The system also maintains detailed access logs for complete traceability.
Still have questions?
Explore how Clinion AI can accelerate your trial – reach out to our team.
Responsible AI
Clinion follows Responsible AI principles, ensuring its AI tools are built for safety and reliability, and remains committed to Data Privacy and Security at every step.
- Accountability
- Transparency
- Privacy & Security
- Reliability & Safety
- Fairness
Unlock the Future of Clinical Trials with Clinion.
Cut your trial costs by 35% and accelerate your time-to-market by 30%
Compliance
Fully Compliant with Global Standards


