CSR-Automation
Clinical Study Report Made Simple with GenAI
Fast. Structured. Regulatory-Ready.
Why Clinion CSR Automation
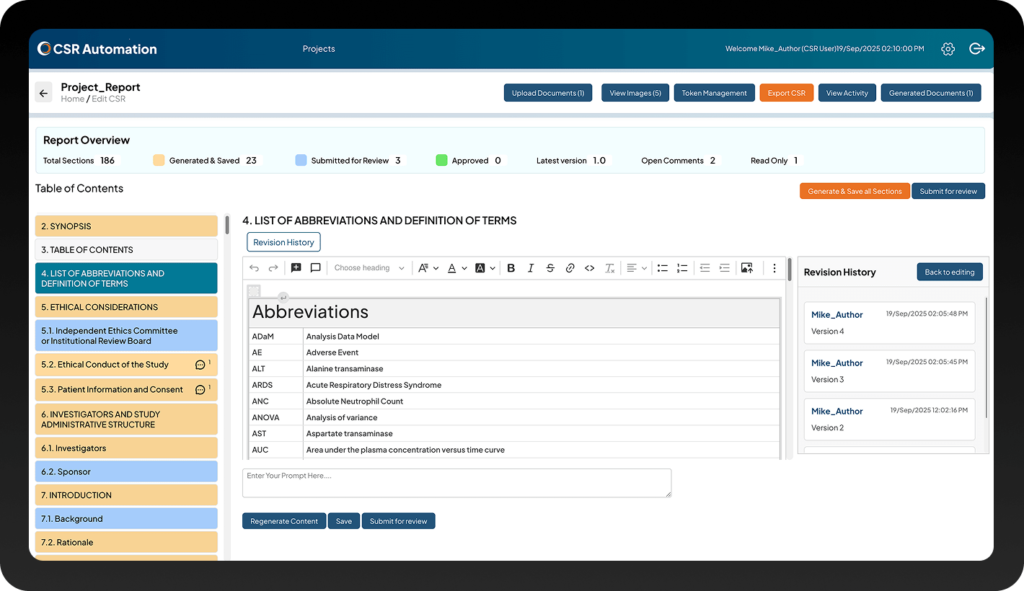
Empower Medical Writers with Submission-Ready Reports
Stop spending weeks manually authoring and compiling Clinical Study Reports. With Clinion’s AI-enabled CSR Automation tool, you can seamlessly extract content from Protocols, Statistical Analysis Plans (SAP), and TLFs. The tool then automatically generates accurate, consistent, and well-structured CSR sections aligned with industry guidelines for your CSR clinical trials, significantly reducing timelines and minimizing the risk of human errors.
CSR Automated
AI extracts key study insights from Protocol, SAP, and TLFs, auto-populating 70% of the Clinical Study Report (CSR) for your CSR clinical study. You can review, edit, and finalize them.
Compliant CSRs
Ensure every Clinical Study Report meets ICH E3 guidelines and global regulatory standards with built-in compliance checks.
Faster Submission
Reduce weeks of manual writing and review, accelerating submission-ready Clinical Study Reports from your CSR clinical trials with unmatched accuracy.
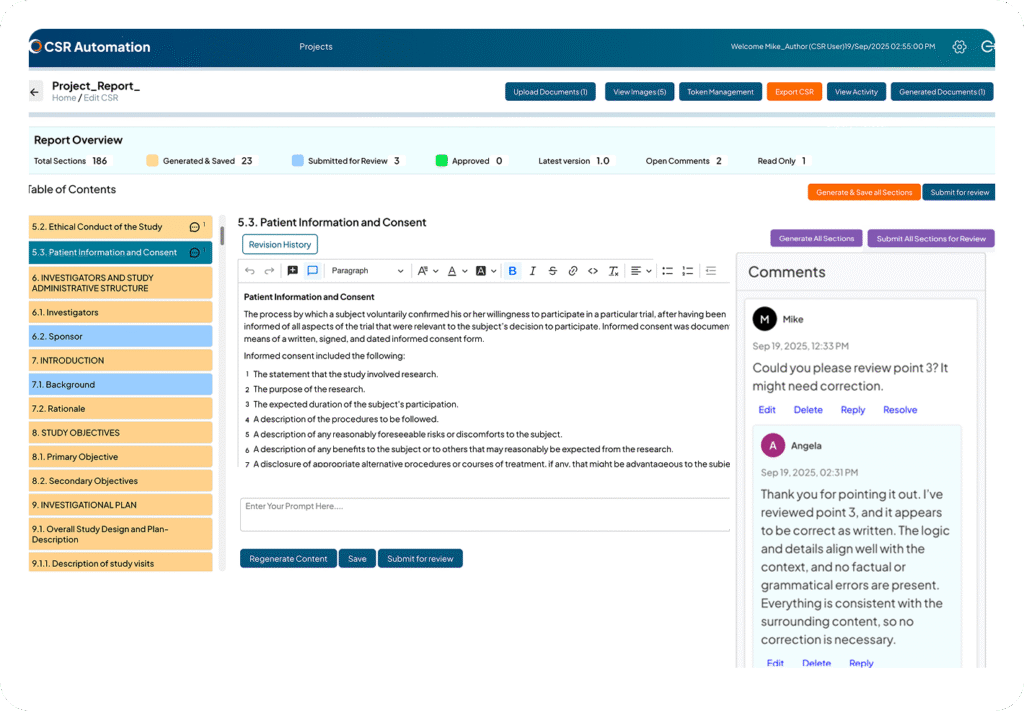
From Data to Draft in Minutes
Upload Study Documents
AI scans Protocol, SAP, and TLFs to extract relevant content.
AI Populates CSR Sections
Automatically fills structured sections of the Clinical Study Report using CSR Automation, ensuring consistency and compliance.
Human Review & Refinement
Medical writers review the AI-generated Clinical Study Report content, provide comments, and refine or regenerate sections as needed.
Submission-Ready CSR
Finalized CSR is fully formatted and structured, ready for regulatory submission.
Features
Key Features for Intelligent CSR Automation
AI‑Generated CSRs
Leverage the power of Generative AI to automatically create your Clinical Study Reports for all your CSR clinical trials. The system intelligently extracts content from Protocol, SAP, and TLFs to generate accurate, well-structured sections, significantly reducing manual writing time and ensuring consistency across the document.
Lifecycle & Version Control
Manage the entire clinical study report lifecycle, from initial draft to final approval, with clear status indicators for each section. The system provides a complete revision history, allowing you to track changes over time. Easily compare versions and access previously generated documents for a fully transparent and auditable process.
Dynamic & Custom Templates
Start with our built-in, ICH E3-compliant template or upload your own template to maintain standardization across every CSR clinical study. Client Admins can manage a library of custom templates and ensure every generated Clinical Study Report adheres to your specific formatting requirements.
Collaboration Tools
Foster seamless collaboration between authors and reviewers within a single platform. Users can add inline comments to specific text, revert sections with clear feedback, and approve content. This iterative workflow ensures all stakeholders are aligned, accelerating review cycles and improving report quality.
Compliance Assurance
Generate reports with confidence, knowing compliance is built into the core of our platform. We provide a default ICH E3-compliant template and allow admins to manage their own standardized templates, ensuring every Clinical Study Report meets global regulatory requirements for submission.
Interactive Demo
Explore CSR Automation
CSR Automation Guided Walkthroughs
Take a guided tour through our end-to-end workflow from each key perspective. See how Admins effortlessly set up projects and manage users, while an Author uses AI to instantly generate a structured CSR draft. Then, step into the Reviewer’s role to add inline comments, track revisions, and approve content, creating a seamless collaborative cycle that produces a compliant, submission-ready report faster than ever.
FAQS
Frequently Asked Questions (FAQ)
Discover quick solutions to your Clinion CSR Automation platform queries
A CSR is submitted after the last patient’s final visit and once all trial data is cleaned and analyzed. It’s typically included in the clinical module of a regulatory submission, such as a New Drug Application (NDA) or Marketing Authorization Application (MAA), and is required for trial closure and regulatory review.
Yes. Clinion helps clinical teams generate submission-ready CSRs up to 70% faster by automating major writing tasks. The AI ensures correct formatting and section structure, enabling quicker internal reviews and fewer revision cycles. This shortens the path from data lock to regulatory submission.
Automation makes CSR writing easier by extracting content from protocol, SAP, and TLFs and automatically populating large parts of the report. Medical writers no longer need to spend hours extracting data or reformatting content. They can simply review and adjust AI-generated sections, making the entire process more efficient and less error-prone.
Yes. Clinion allows full flexibility to review, edit, and regenerate any AI-populated section of the CSR. Medical writers can adjust the content to match study-specific context or formatting needs. This ensures that while automation speeds up the process, you stay in control of the final report's accuracy and compliance.
Intelligent mapping in Clinion refers to how the AI links specific content from study documents to corresponding CSR sections. This ensures that trial data is accurately placed without manual tagging. It supports both automated input and manual overrides when needed.
An end-of-study report provides a brief overview confirming the completion of a clinical trial, often required for registry databases. In contrast, a CSR offers a full, detailed analysis of trial data for regulatory review. While both mark trial completion, only the CSR is used for formal submissions and drug approval.
Still have questions?
Explore how Clinion AI can accelerate your trial – reach out to our team.
Responsible AI
Clinion follows Responsible AI principles, ensuring its AI tools are built for safety and reliability, and remains committed to Data Privacy and Security at every step.
- Accountability
- Transparency
- Privacy & Security
- Reliability & Safety
- Fairness
Unlock the Future of Clinical Trials with Clinion.
Cut your trial costs by 35% and accelerate your time-to-market by 30%
Compliance
Fully Compliant with Global Standards


